Research Overview


Former Resnick Fellow Cody Finke and RSI Executive Director for Programs Neil Fromer are a part of a team that developed a model to analyze the real production costs and carbon emissions from the production of industrial hydrogen. The team shows that sulfur electrolysis (SE) to produce hydrogen and sulfuric acid, has the potential to produce up to 36% of global hydrogen demand under current economic conditions, while water electrolysis (WE) is not economical under today's conditions. They identify criteria for economic hydrogen production and find potential reactions to produce more than 150% of current global hydrogen production. The results can be found in their recent publication, "Economically advantageous pathways for reducing greenhouse gas emissions from industrial hydrogen production under common, current economic conditions."
Significance and Impact
There are methods of producing hydrogen, such as sulfur electrolysis (coproduction of hydrogen and sulfuric acid) that could be cheaper and cleaner than steam methane reforming. Water splitting may not be one of them.


Technical Details
- The model calculates levelized cost of hydrogen based on real data for capital and operating costs.
- While the high energy inputs and lack of a co-product make water splitting uneconomic, we identify several reactions that together could meet global hydrogen demand.
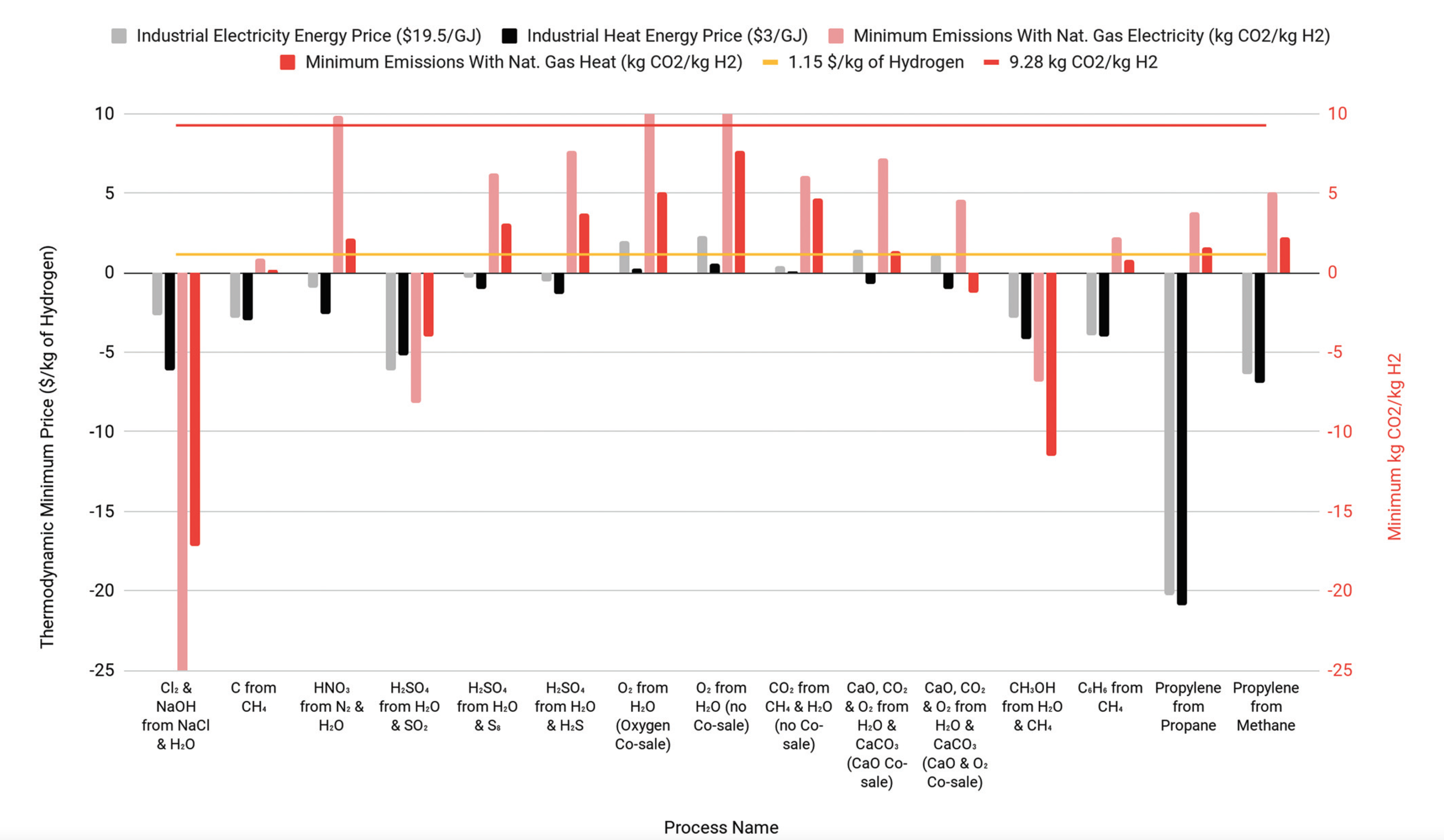

Cody E. Finke, Hugo F. Leandri, Evody Tshijik Karumb, David Zheng, Michael R. Hoffmann, and Neil A. Fromer, Economically advantageous pathways for reducing greenhouse gas emissions from industrial hydrogen production under common, current economic conditions. Energy & Environmental Science (2021). https://doi.org/10.1039/d0ee03768k
Contact: Neil Fromer

